In the face of climate change, the world is currently facing the great challenge of ensuring adequate and healthy nutrition. In doing so, environmental aspects must be taken into account that keeps an eye on local production limits as well as global supply chains. Since it is forseeable that vegetation areas for food production will increasingly shrink due to land consumption and climate change, sustainable management of the soil and marine areas is necessary. The quality of the food is another issue that has to be discussed in terms of animal welfare and productivity, among other things.
For all these questions, a transnational consortium of different research institutions from eight European countries (Ita, Nor, Por, Esp, Fra, Deu, Bel, and Let) was formed in July 2020, which uses national research funds to promote the exchange of ideas and close knowledge gaps regarding the problems outlined above. The European Joint Programming Initiative (JPI) association has taken the name SYSTEMIC to work on an "integrated approach to the challenge of sustainable food". The ZIEL Institute for Food and Health at the Technical University of Munich contributes with its expertise in healthy nutrition and the implementation of human intervention studies to generate knowledge in two of seven work packages. Please find further information and opportunities for cooperation via the link http://systemic-hub.eu/.
Many of today's widespread so-called civilisation diseases have one common risk factor: overweight and obesity. The resulting metabolic diseases, such as diabetes mellitus, hypertension and arteriosclerosis, place a great burden on those affected and restrict their everyday activity.
Hunger and satiation are important factors that determine the development of overweight. It is known that carbohydrates, fats and proteins have different effects on satiation. However, the hormonal mechanisms behind this are still not completely understood. In addition, a rediscovered tissue type is attracting increasing interest. The so-called brown adipose tissue (BAT) is able to release energy directly in the form of heat. This offers a possibility to counteract the development of overweight by means of increased heat production. The project funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) is now researching if and how this fatty tissue can be activated by a defined nutritional intervention.
For this goal a collaboration of different institutes of the Technical University of Munich (TUM) have found the IMAGO (Non-invasive imaging of tissue plasticity and adaption in response to metabolic challenges) research group. The iMAGO develops and expands new non-invasive methods to visualise metabolic processes and to better diagnose and treat people with obesity.
In the study centre of the ZIEL - Institute for Food and Health at TUM, the meal-dependent reaction of secretin release and the activity of brown adipose tissue are being investigated.
In the first stage, different meal types will be tested to see how secretin release and associated thermogenesis vary. Non-invasive imaging techniques will be used for this.
We are recruiting healthy volunteers (caucasien, female and male)
- age: older than 18
- BMI > 27 kg/m2
LINK IMAGO: www.imago-for5298.de
The study XcellentDiet investigates the accuracy of the app of the same name in capturing calorie and nutrient information compared to reference values. The app utilizes image recognition, text, and voice input to simplify dietary documentation. Thirty participants will record their meals over four days using weighed food records and photos taken from two perspectives. These data will be analyzed using the dietary software “OptiDiet®” as well as other nutrition-related apps (e.g., Cal AI) to assess accuracy compared to established methods. In addition, the consistency of image recognition will be evaluated across different camera models, photo angles, and repeated analyses. The speech function will be tested by entering dietary records via voice input. The aim is to validate the reliability and efficiency of XcellentDiet as an innovative tool for dietary assessment.
Further information can be found on the DRKS website or in our flyer
We are hiring software/AI Engineers!

Allulose as a sugar substitute not only for diabetes
Allulose is a naturally occurring sugar that has a slightly lower sweetening power than table sugar and hardly differs in taste. Due to its properties, allulose can be used as a sugar substitute in energy-reduced foods and beverages.
It is already known from initial studies that allulose is only metabolized in very small quantities and is excreted virtually unchanged via the kidneys. As a result, allulose provides hardly any energy and its consumption does not cause a rise in blood glucose levels. For diabetics, this has the advantage that the body does not need insulin to process the allulose. For weight problems, the consumption of allulose-sweetened foods can have a positive effect on body weight.
Since allulose, which has received GRAS (generally recognized as safe) status in the U.S. and Asia, has applied for EU approval, further studies are needed on which to base this approval. The Core Facility for Human Studies of the ZIEL - Institute for Food and Health at TUM is investigating the dose-dependent uptake, metabolism and excretion of allulose as part of such a registration study. Equally, the evaluation of tolerability and the occurrence of possible side effects, such as gastrointestinal complaints, is of interest.

Overweight and obesity lead to metabolic diseases such as diabetes mellitus type 2 or dyslipidemia and subsequently to increased CHD or strokes. Guidelines recommend reducing body weight to prevent subsequent damage.
Among the various strategies, 16/8 interval fasting represents a non-restrictive method that allows the total intake of daily calories during an 8-hour period while being shown to be associated with moderate body weight reduction. However, the fasting period of 16 hours carries the risk that a strong feeling of hunger may counteract the potential success of interval fasting. To prevent this, however, the increased intake of additional dietary fiber could represent a solution strategy, since dietary fiber has been shown to promote satiety.
Within the framework of the DFG application of Dr. Beate Brandl, we want to investigate the effect of dietary fiber on satiety in a 16/8 intermittent fasting trial in a study. Over 12 weeks, the amount of dietary fiber will be increased by 40 g/day under everyday conditions. With the help of the data, we hope to be able to clarify the question of whether fiber-enriched foods support a longer-lasting feeling of satiety during 16/8 intermittent fasting, so that weight can be reduced more easily and thus the CHD risk and the risk of developing type 2 diabetes mellitus can be lowered.
Micronutrients are trace elements and vitamins that are essentially absorbed by humans from food and are essential for many physiological functions. Nevertheless, around two billion people worldwide - including Germany - are affected by a micronutrient deficiency. The causes of a deficiency supply are manifold and range from regional soil conditions to malnutrition or an unbalanced diet.
An important property of micronutrients, such as selenium, vitamin D or zinc is their role in the immune system, especially in immune defense. Many studies have already established links between deficiencies of these nutrients and increased risk of bacterial and viral infectious diseases, possibly including SARS-CoV-2 infection.
Therefore, as part of the EIT (European Institute of Technology) Crisis Response Initiative, the MeDiCo-Health (Micro-nutrient Deficiency in Covid-19 patients and Health care professionals) study was launched in response to the current Covid-19 pandemic. Among other things, the MeDiCo-Health study seeks to determine whether the micronutrient status and eating patterns of individuals infected with SARS-CoV-2 have an impact on disease progression.

In nutritional research, the quality of the nutritional survey is of great importance in order to be able to examine associations between nutrition or nutrients and health in larger cohorts. The reliable recording of the intake of food, nutrients or food contaminants is a major challenge, because inaccuracies in the currently available survey methods (questionnaires) often deliver distorted or misleading results [1,2]. Biomarkers for the consumption of different foods, food groups or ingredients can be an objective measure of intake and as a supplement to the classic methods of nutrition research [3,4]. However, to date there are only a few food biomarkers that have been validated. Although there are established concepts for identifying disease biomarkers in medicine, there are still no clear recommendations for the development of biomarkers of the nutritional status. Many markers have been proposed, but few are sufficiently validated and considered representative of food intake and/or for determining nutritional status [5,6]. The aim of this study was, therefore, to identify possible biomarkers for selected foods (beef, chicken meat). To do this, we collected blood and urine samples at defined times before and after a test meal and analyzed the broadest possible spectrum of metabolites in the blood and urine. By taking repeated samples over a period of up to 48 hours, we determined the biokinetics of the food ingredients or their metabolites. Using bioinformatic and biostatistical methods, we were able to determine a small spectrum of factors that indicate meat consumption (e.g. 𝝅-methylhistidine, trimethylamine-N-oxide (TMAO), dimethylglycine). However, questions about the specificity of the markers still remain unanswered.
The results have now been published and discussed in a publication in the journal "Molecular Nutrition & Food Research":
Giesbertz P. & Brandl B. et al. Specificity, Dose Dependency, and Kinetics of Markers of Chicken and Beef Intake Using Targeted Quantitative LC-MS/MS: a Human Intervention Trial. Mol Nutr Food Res. 2020 Jan 9:e1900921: https://onlinelibrary.wiley.com/doi/10.1002/mnfr.201900921
Literature
[1] Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Human genetics 2009; 125(5‐6): 507‐25.
[2] Penn L, Boeing H, Boushey CJ, et al. Assessment of dietary intake: NuGO symposium report. Genes & nutrition 2010; 5(3): 205‐13.
[3] Scalbert A, Brennan L, Manach C, et al. The food metabolome: a window over dietary exposure. The American journal of clinical nutrition 2014; 99(6): 1286‐308.
[4] Garcia‐Aloy M, Llorach R, Urpi‐Sarda M, et al. Novel multi‐metabolite prediction of walnut consumption by a urinary biomarker model in a free‐living population. Journal of proteome research 2014.
[5] Hjerpsted J, Ritz C, Schou S, Tholstrup T, Dragsted L. Effect of cheese and butter intake on metabolites in urine using an untargeted metabolomics approach. Metabolomics 2014: 1‐10.
[6] Stanstrup J, Schou SS, Holmer‐Jensen J, Hermansen K, Dragsted LO. Whey Protein Delays Gastric Emptying and Suppresses Plasma Fatty Acids and Their Metabolites Compared to Casein, Gluten, and Fish Protein. Journal of proteome research 2014.
Trimethylamine N-oxide (TMAO) is a biomarker for fish, meat, and egg intake [1, 2]. Several studies suggest an association between elevated plasma TMAO levels and increased risk of cardiovascular disease [3, 4]. In the meatmark study, we investigated this biomarker in more detail.
Beside the natural occurrence of TMAO in foods, e.g. fish, TMAO can be produced in the human body itself. First, some microorganisms in the intestine produce the precursor of TMAO, TMA (trimethylamine), from the dietary precursors choline, betaine and L-carnitine. In a second step, TMA is enzymatically converted to TMAO in the liver. The TMAO value in plasma, therefore, depends on diet as well as on the composition and activity of the gut microbiome [3,4].
The aim of the meatmark study was, on the one hand, to investigate whether a dietary fibre supplementation can modulate the gut microbiome to affect the production of TMA/TMAO after beef consumption. On the other hand, two genes of TMAO metabolism were investigated for mutations and their effect on plasma TMAO levels.
For the study, 14 healthy subjects (7 females, 7 males) aged 18 to 40 years were recruited. In a randomized cross-over double-blind study, the volunteers consumed 200 g of beef during a test meal. This beef intervention was preceded by two weeks of double blinded dietary fibre supplementation (with a dietary fibre or placebo supplement). At defined time points before and after the intervention, blood, urine, and stool samples were collected and analysed using various analytical methods.
Literature
[1] E. M. Brouwer-Brolsma, L. Brennan, C. A. Drevon, H. van Kranen, C. Manach, L. O. Dragsted, H. M. Roche, C. Andres-Lacueva, S. J. L. Bakker, J. Bouwman et al., The Proceedings of the Nutrition Society, DOI: 10.1017/S0029665117003949 .
[2] A. Scalbert, L. Brennan, C. Manach, C. Andres-Lacueva, L. O. Dragsted, J. Draper, S. M. Rappaport, J. J. J. van der Hooft, D. S. Wishart, The American journal of clinical nutrition, DOI: 10.3945/ajcn.113.076133 .
[3] Ulaszewska MM, Weinert CH, Trimigno A, Portmann R, Lacueva CA, Badertscher R, Brennan L, Brunius C, Bub A, Capozzi F, Rosso MC, Cordero CE, Daniel H, Durand S, Egert B, Ferrario PG, Feskens EJM, Franceschi P, Garcia-Aloy M, Giacomoni F, Giesbertz P, Domínguez RG, Hanhineva K, Hemeryck LY, Kopka J, Kulling S, Llorach R, Manach C, Mattivi F, Migné C, Münger LH, Ott B, Picone G, Pimentel G, Pujos-Guillot E, Riccadonna S, Rist M, Rombouts C, Rubert J, Skurk T, Sri Harsha PSC, van Meulebroek L, Vanhaecke L, Vázquez-Fresno R, Wishard D, and Vergères G. Nutrimetabolomics: An Integrative Action for Metabolomic Analyses in Human Nutritional Studies. Mol Nutr Food Res 2018:e1800384. Doi: http://dx.doi.org/10.1002/mnfr.201800384<
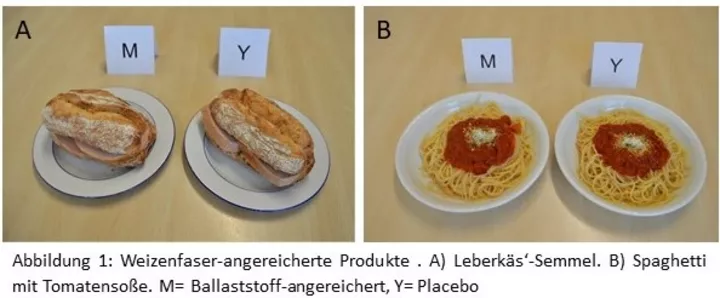
A high-fiber diet has a positive effect on satiety, regulates digestion, promotes intestinal health [1], and reduces the risk of numerous diet-related diseases such as type 2 diabetes mellitus and arteriosclerosis, which can lead to heart attacks and strokes [2, 3, 4]. Some positive effects, including intestinal health, can be attributed to the increase in stool volume [5, 6, 7] and the reduced transit time [5]. Fiber prevents constipation, but also reduces the risk of cardiovascular disease, diabetes mellitus, and dyslipidemia [8, 9]. With adequate fluid intake, the shorter transit time reduces hard stool consistency and, thus, the risk of diverticulosis [10], which can lead to frequent inflammation and intestinal perforation. In addition, some studies indicate that dietary fiber also binds bile acids, which prevents them from being transported back into the blood in the small intestine and increases bile acid synthesis. Because of this, some authors attribute a cholesterol-lowering effect to dietary fiber [11, 12, 13, 14]. The increase in the amount of stool by adding fiber in the form of sugar beet fiber, barley fiber, and oat grain fiber to the diet has already been shown and has been provided with a health claim [14, 6]. This means that health-related statements can be made in advertising.
In the present study, the focus was placed on wheat fiber with regard to its increase in stool volume. To investigate the effect of wheat fiber on stool volume, subjects were randomly blinded to either wheat fiber-enriched foods, wheat fiber-enriched beverages, or the respective placebo without fiber in a randomized, cross-over design. In cooperation with an industrial partner, we developed wheat fiber-enriched foods and a wheat fiber-enriched drink. In the active dietary fiber intervention, the subjects received 10 g of insoluble dietary fiber in the form of a wheat fiber per day in addition to their usual diet. In order to make the dietary fiber intake close to everyday life and physiological, a total of 10 g wheat fiber/day (3x 3.33 g wheat fiber/portion) was offered as a drink or food. During the placebo phases, subjects received the same products without the fiber but a filler. At defined times before, during, and after the intervention, the amount of stool was recorded both fresh and freeze-dried. In order to analyze as broad a spectrum as possible of the effect of the increased intake of wheat fiber on human physiology, urine samples were collected in addition to the stool samples.
The detailed results were published as part of a publication in the journal "Nutrients":
Brandl et al. Effects of Extrinsic Wheat Fiber Supplementation on Fecal Weight; A Randomized Controlled Trial. Nutriens. 2020, 12, 298.
Literature
[1] Cummings JH. The effect of dietary fiber on faecal weight and composition. Spiller GA, ed. CRC handbook of dietary fiber in human nutrition, 2nd edn. Boca Raton: CRC Press, 1993; 263–349.
[2] Trowell H. Ischemic heart disease and dietary fiber. American Journal of Clinical Nutrition 1972; 25: 926–932.
[3] Southgate DAT. Dietary fibre and the diseases of affluence. In a Balanced Diet 1988; 117–139.
[4] Chen K, Qiu JL, Zhang Y, Zhao YW. Metaanalysis of risk factors for colorectal cancer. World J Gastroenterol. 2003 Jul 15; 9(7): 1598–1600.
[5] Drasar BS, Jenkins DJA, Cummings JH. The influence of a diet rich in wheat fibre on the human faecal flora. Journal of Medical Microbiology 1976; Vol 9.
[6] Chen HL, Haack VS, Janecks CW, Vollendorf NW, Marlett JA. Mechanisms by which wheat bran and oat bran increase stool weight in humans. American Journal of Clinical Nutrition 1998; 68:711-9.
[7] Stephen AM, Wiggins HS, Englyst HN, Cole TJ, Wayman BJ, Cummings JH. The effect of age, sex and level of intake of dietary fibre from wheat on large-bowel function in thirty healthy subjects. British Journal of Nutrition 1986; 56: 349-361.
[8] Monro JA. Wheat bran equavilents based on faecal bulking indices for dietary management of faecal bulk. Asia Pacific Journal Clinical Nutrition 2001; 10 (3): 242-248.
[9] Lairon D, Arnault N, Betrais S, Planells R, Clero E, Herchberg S, Boutron-Ruault MC. Dietary fibrer intake and risk factors for cardiovascular disease in French adults. American Journal of Clinical Nutrition 2005;82:1185-94.
[10] Aldoori WH: The protective role of dietary fiber in diverticular disease. Adv Exp Med Biology 1997; 427; 291–308.
[11] Brown L, Rosner B, Willet WW, Sacks FM. Cholesterol-lowering effects of dietary fiber - A meta-analysis. American Journal of Clinical Nutrition 1999; 69: 30.
[12] Truswell AS, Beynen, AC. Dietary fibre and plasma lipids: Potential for prevention and treatment of hyperlipidemias. Dietary Fibre - a Component of Food 1992; 295-332.
[13] Truswell AS. Meta-analysis of the cholesterol-lowering effects of dietary fibre. American Journal of Clinical Nutrition. 1999; 70(5):942-3.
[14] Langkilde AM, Andersson H, Bosaus I. Sugar-beet fibre increase cholesterol and reduces bile acid excretion from the small bowel. British Journal of Nutrition 1993; 70: 757-766.